Canada Approves At-home Entyvio Injection for Crohn’s

Health Canada has approved a self-injectable subcutaneous (under-the-skin) form of Entyvio (vedolizumab) as an at-home maintenance therapy for adults with Crohn’s disease (CD).
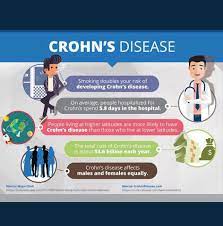
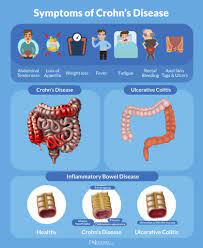
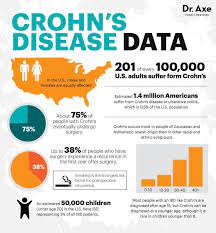
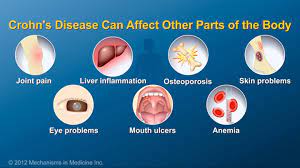
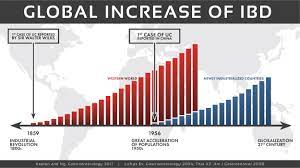
The approval follows an earlier decision to authorize the medication for ulcerative colitis. These two conditions comprise the main forms of inflammatory bowel disease (IBD), of which Canada has the world’s highest prevalence.
“Having a formulation of vedolizumab that can be self-administered is an important new delivery method for IBD patients with Crohn’s disease or ulcerative colitis,” Brian Bressler, MD, director of the Advanced IBD Training Program and professor at the University of British Columbia, said in a press release. “Some patients prefer this option because it gives them greater control over when and where they receive their treatment.”
Entyvio is a monoclonal antibody, developed by Takeda Pharmaceuticals, that treats CD and ulcerative colitis by preventing inflammatory cells from entering the gut. Until now, the therapy has been available only as an intravenous (into-the-vein) injection.
The new subcutaneous formulation — injected by pre-filled syringe or pen — makes at-home treatment easier as one can safely self-administer the therapy without a great deal of expertise.
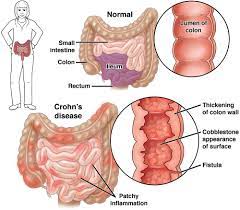
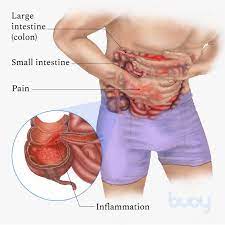
Entyvio is intended for use in adults with moderately to severely active CD, who do not respond or cannot use immunomodulators, tumor necrosis factor-alpha antagonists, or corticosteroids.
The new formulation is designed for use after at least two intravenous infusions. Patients who respond well to this treatment may switch to the new formulation. The first subcutaneous dose occurs in place of a scheduled intravenous dose, followed by a dose every two weeks.
The medication demonstrated positive safety and efficacy in two Phase 3 trials — VISIBLE 1 (NCT02611830) in ulcerative colitis and VISIBLE 2 (NCT02611817) in CD — that collectively evaluated more than 1,000 volunteers. One year of treatment in these trials led to remission rates of 46.2% in ulcerative colitis and 48% in CD.
These results, as well as those from an ongoing open-label extension study (NCT02620046), contributed to approval in the European Union of the new subcutaneous formulation last year.
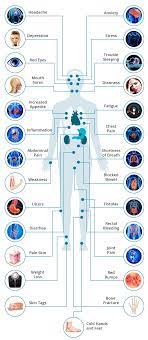
“People living with chronic conditions like Crohn’s disease or ulcerative colitis face many challenges in managing their disease,” Susan Cowan, CEO of Crohn’s and Colitis Canada, said. “Having options in treatment approaches gives people more freedom in making choices that best meet their needs.”
“The ability to self-administer medication in a home setting is especially relevant during the COVID-19 pandemic for people looking to reduce time spent outside the home,” she added.
The post Canada Approves At-home Entyvio Injection for Crohn’s appeared first on IBD News Today.