FDA clears IND for clinical trial testing switchable CAR-T therapy in patients with autoimmune diseases, without chemotherapy

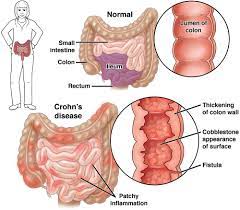
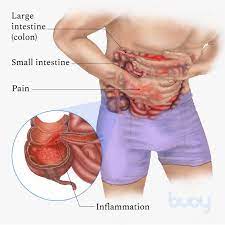
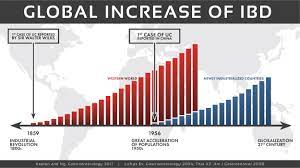
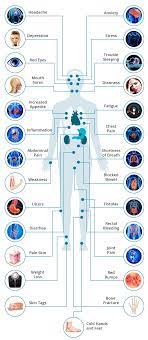
FDA has cleared an investigational new drug (IND) application to study switchable chimeric antigen receptor T cell (sCAR-T) therapy (CLBR001 + SWI019) in patients with autoimmune conditions. Patient recruitment for the phase 1 trial will begin soon (NCT06913608). The phase 1 clinical trial will evaluate the safety and efficacy of CLBR001 + SWI019 in patients with myositis, systemic sclerosis, lupus and rheumatoid arthritis, with the potential to expand to other indications in the future.