ICN Research Explained: Biosimilars for Pediatric Patients With Inflammatory Bowel Disease: Pediatric Gastroenterology Clinical Practice Survey


Why was this study done?
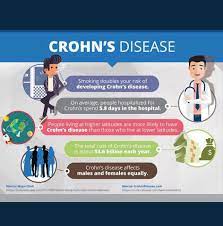
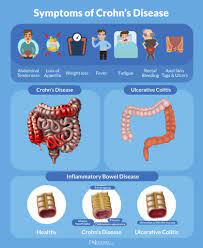
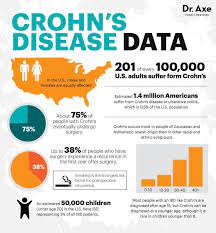
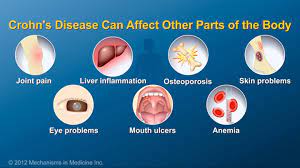
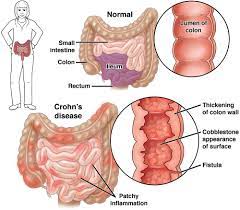
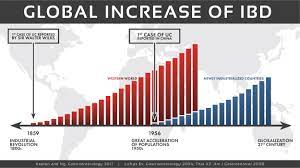
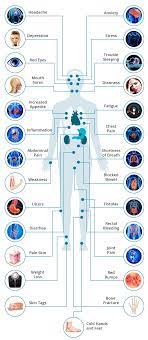
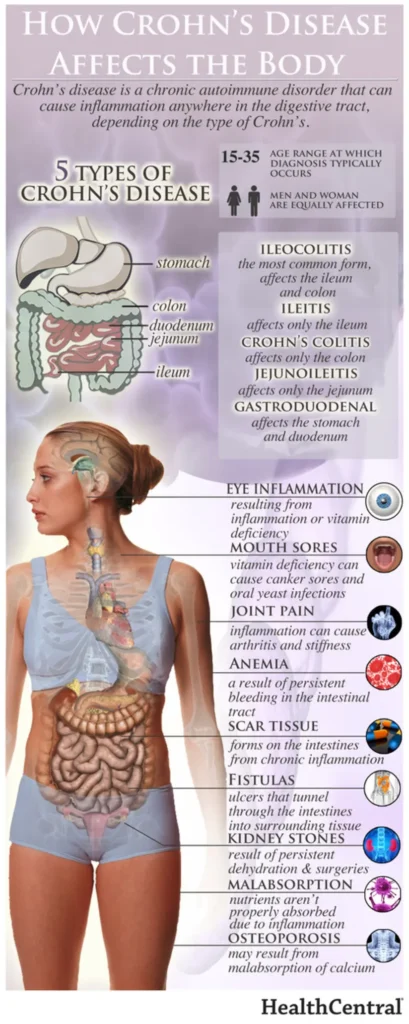
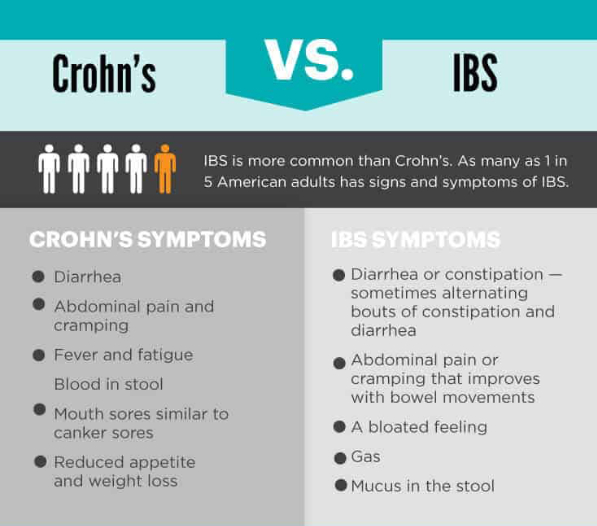
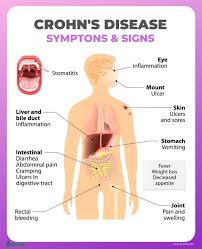
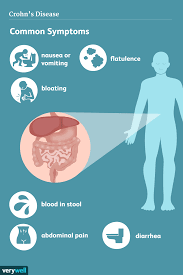
Pediatric patients with inflammatory bowel disease (IBD), including Crohn’s Disease and ulcerative colitis, who take biologic medications (like infliximab and adalimumab) have decreased hospitalizations, surgery rates, and improved quality of life. However, biologic medicines are expensive. Biosimilars are similar biological therapies that are just as safe and effective as the original biologic medication, although they are often less expensive. There are currently two medications (infliximab/Remicade and adalimumab/Humira) used to treat IBD that have biosimilars available. Despite the cost savings, the utilization of biosimilars in the treatment of IBD has been low. The exact reasons why the utilization of biosimilars is low is not well known or well-studied.
The objective of this study was to evaluate pediatric gastroenterologists’ perspectives of biosimilars and to evaluate factors that impact pediatric gastroenterologists’ comfort level with prescribing biosimilars.