Phage Therapy BX002 Shows Promise in First-in-human Trial

BX002, an oral phage therapy BiomX is developing for inflammatory bowel disease (IBD), showed a good safety profile, as well as the ability to effectively deliver its active agent to the gut, in a first-in-human clinical trial, the company announced in a press release.
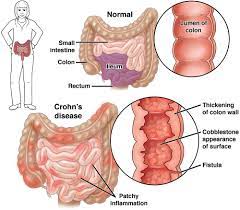
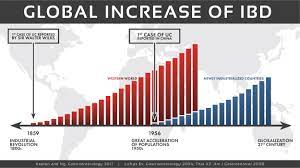
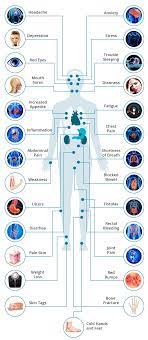
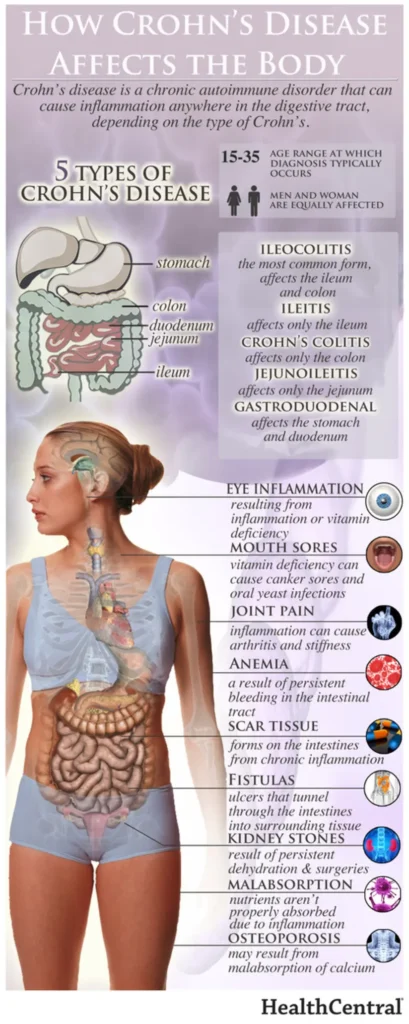
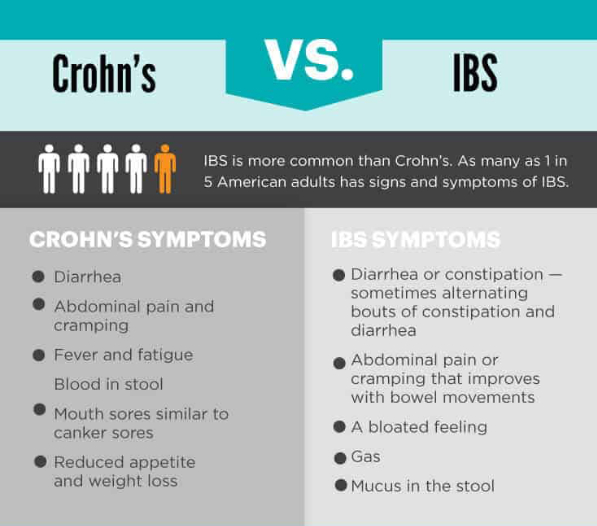
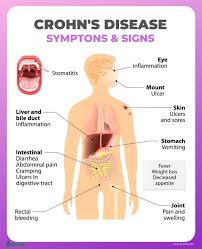
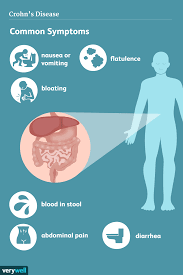
The human gut is home to billions of bacteria — the gut microbiome — and these tiny organisms play an important part in human health. Abnormalities in the gut microbiome have been implicated in the development of IBD.
Phages, short for bacteriophages, are viruses that can infect and kill bacterial cells. Different types of phages can infect different types of bacteria, depending on the exact biology of each.
Using a specific mix of phages, BX002 aims to target and kill a type of bacteria known as Klebsiella pneumoniae, more simply called K. pneumoniae. This bacteria is thought to be a cause of IBD and another chronic condition called primary sclerosing cholangitis (PSC), a disease that affects the bile ducts.
BiomX sponsored a Phase 1 clinical trial (NCT04737876) to evaluate BX002 in 18 healthy volunteers. The investigational therapy was given to 14 participants twice daily for three days; the other four participants were instead given a placebo. All of the participants were monitored for a week, with additional follow-up safety assessments done at two and four weeks after treatment.
The study’s main goal was to test the investigational therapy’s safety and tolerability. Overall, its safety-related results were positive, as there were no serious adverse events reported. There also were no adverse events that caused participants to discontinue the study.
The results also demonstrated that the oral medication was able to deliver approximately 10 billion plaque-forming units of phage into the gut — that’s about 1,000 times more than the amount of K. pneumoniae bacteria found in the stool of people with IBD or PSC, according to BiomX.
“Successful oral delivery of phage therapy, which to our knowledge has now been demonstrated rigorously in a clinical study for the first time, has the potential to open up a broad range of oral phage therapy applications,” said Timothy K. Lu, MD, PhD, a professor at the Massachusetts Institute of Technology and scientific co-founder at BiomX.
“The results show excellent safety, as expected, and the pharmacokinetics [the movement of drug into, through, and out of the body] demonstrate the potential to orally administer phage in a quantity sufficient to address the gut bacterial burden of K. pneumoniae in IBD and PSC patients,” Lu added.
Based on the positive results, BiomX is now planning a Phase 1b/2a study to evaluate a similar oral phage therapy called BX003 in people who have K. pneumoniae bacteria in their guts. Results from this trial are expected by mid-2022.
The post Phage Therapy BX002 Shows Promise in First-in-human Trial appeared first on IBD News Today.