Yuflyma, Biosimilar to Humira, Approved in EU for Chronic Inflammatory Diseases

The European Commission (EC) has granted marketing authorization to Celltrion Healthcare‘s Yuflyma (CT-P17), a biosimilar to Humira (adalimumab).
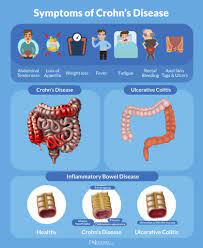
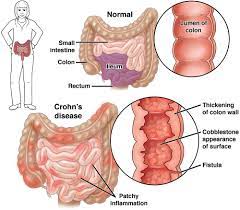
The marketing authorization includes all indications for which AbbVie’s Humira is approved, including adult and pediatric Crohn’s disease and ulcerative colitis, two forms of inflammatory bowel disease (IBD).
Biosimilars are biological products with similar properties to an original product — in this case, Humira — but manufactured by a different company. Of note, a biological product is made from living cells rather than by chemical production.
Biosimilars have marketing approval after patent expiration of the original products, and are usually marketed as cheaper versions of brand name therapies, emerging as powerful products for cost savings in healthcare systems.
Adalimumab, the active ingredient in both Yuflyma and Humira, helps reduce inflammation by targeting and blocking the pro-inflammatory molecule tumor necrosis factor.
“Over the past two decades, anti-tumor necrosis factor (TNF) biologics have revolutionized the management of chronic immune-mediated inflammatory diseases, but some of the features needed improvement for patients to reach their therapeutic goals,” Rieke Alten, MD, PhD, a department head at Schlosspark-Klinik, Teaching Hospital of Charité, Berlin, Germany, said in a press release.
Yuflyma (also known as CT-P17) is the first high-concentration, low-volume, and citrate-free adalimumab biosimilar. According to Celltrion, these characteristics reduce injection discomfort, as citrate was found to cause pain upon injection (of note, Humira’s formulation contains citrate).
“With high concentration, low-volume, and consequently less pain, adalimumab can improve treatment adherence at the very least,” said HoUng Kim, PhD, head of the medical and marketing division at Celltrion Healthcare. “Therefore, we focused on development of a high concentration biosimilar to provide a significant alternative to the adalimumab treatment category.”
Moreover, Yuflyma is delivered through a fine needle (29G size) and a latex-free device to reduce the risk of allergic reactions, being a more convenient option for patients, according to Celltrion. The therapy has a long storage period of up to 24 months at 2 C to 8 C, or up to 30 days at room temperature.
Yuflyma’s approval in the EU follows a positive recommendation in December from the Committee for Medicinal Products for Human Use of the European Medicines Agency.
The approval was based on results of studies that showed Yuflyma’s biosimilarity to Humira in terms of safety, efficacy, and immunogenicity (ability to trigger an immune reaction).
Like Humira, Yuflyma was approved to treat not only adult and pediatric Crohn’s disease and ulcerative colitis, but also 10 other types of inflammatory diseases, including rheumatoid arthritis, psoriatic arthritis, plaque psoriasis, ankylosing spondylitis, juvenile idiopathic arthritis, among others.
The EC approval is applicable across all 28 European Union member countries as well as Norway, Iceland, and Liechtenstein.
Celltrion plans to seek a marketing authorization of Yuflyma in the U.K. as well.
The post Yuflyma, Biosimilar to Humira, Approved in EU for Chronic Inflammatory Diseases appeared first on IBD News Today.